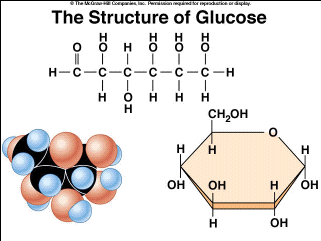
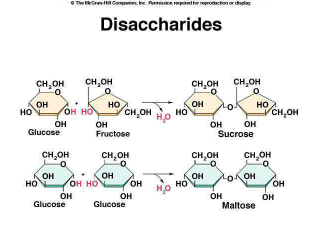
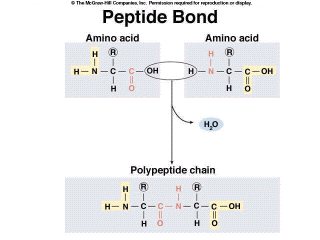
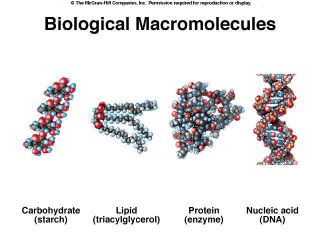A Single Origin of Life
At some point between 4 billion and 3.5 billion years ago, Earth was
transformed from a non-living system to a living system. This time is
constrained by the cooling of the Earth's crust. There was probably
no constant surface water before about 4 billion years. The fossil record
indicates that by 3.5 billion years there were prokaryotes on Earth.
The evidence suggests that all life today had a single common ancestor,
or a single origin. The most compelling argument for this is that all
organisms use the same triplets of nucleotides to code for amino acids.
This universality of the genetic code across all known organisms indicates
a single common ancestor.
Early Conditions on Earth
The early Earth was very different from the planet we a familiar with.
The surface would have been a chaotic place, with strong volcanic activity.
Volcanoes today produce large amounts of water vapor and carbon dioxide,
and it is assumed that the same would have been true early in Earth's
history. Earth also would have experienced heavy bombardment by bolides,
extraterrestrial objects such as asteroids and meteorites. While those
impact craters are no longer apparent today, we only have to look at
the surface of the moon to see evidence that bolide impacts were common
in the early solar system. The atmosphere of early Earth also would
have been very different. It would have been composed primarily of nitrogen,
water vapor, carbon dioxide, with trace amounts of carbon monoxide,
hydrogen and methane. Perhaps most importantly, there would have been
little or no free oxygen. Therefore, the earliest forms of life were
most likely anaerobic, that is they did not require oxygen for
their cellular metabolism. Energy sources on the early earth would have
been lightning, which is produced during volcanic eruptions, and sunlight.
While the sun was weaker at that time, there was no protective ozone
layer in the atmosphere; therefore more ultraviolet (UV) radiation would
have struck Earth's surface.
The Miller-Urey Experiments
In 1953, Stanley Miller, then a graduate student, and Harold Urey, devised
an experiment that would cast new light on the origin of life. They set up an apparatus
that simulated the atmosphere of early Earth. They then applied energy,
in the form of an electric spark, and collected the chemicals that resulted
from this reaction. They were able to synthesize a number of organic molecules
from inorganic components, including some amino acids. Their experiments
have come under scrutiny in recent years because the "atmosphere" that
they used was more H rich than the early Earth's atmosphere is now believed
to be. But, if this experiment is run using a less reducing atmosphere,
biological molecules or their precursors are synthesized. Formaldehyde
(H2CO) is produced, and sugars can be
derived from this molecule. Nucleotide bases can be synthesized from HCN,
which is derived from methane. Finally, some molecules, such as phosphate,
could come from the weathering of rock. But, it is clear that these experiments
have shown that biotic compounds can be synthesized from abiotic compounds.
Where did life originate?
This question of where life originated is very difficult to address.
Darwin spoke of a warm little pond as home for the first life forms.
The support for this is that organic molecules can be synthesized from
a simulated early atmosphere. It has been suggested that these compounds
could rain down into pools, where further biochemical reactions would
then occur. However, there are a number of arguments against this scenario.
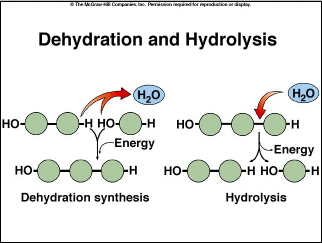
Remember, in our discussion of polymerization, that many of those reactions
are a dehydration synthesis, with the loss of a water molecule.
Some biochemists argue that these reactions would have been unlikely
to occur spontaneously in the water. Also, the surface of the early
earth was most likely repeatedly sterilized by bolide impacts. As mentioned
above, we can see impact craters on the surface of the moon. Some are
very large. The Mare Imbrium crater was caused by a bolide about 70
miles in diameter.
Recently, some researchers have argued that life may have originated
at deep-sea
hydrothermal vents. Today, these vents house a diverse array of
organisms whose ecosystem is based on chemosynthesis, rather than photosynthesis.
Proponents of this theory point to DNA sequence information from organisms
that live today. The data shows that many of the most divergent (oldest)
lineages within the bacteria and archaea are thermophiles, species
that live in very hot environments. However, this also could be the
result of a selective event early in the history of life.
Finally, some researchers have suggested that earth was "seeded" by complex
organic molecules from space. This theory is called panspermia.
In 1969, a meteorite fell near Murchison, Australia. When that meteorite
was examined, it was found to carry many organic chemicals, including
amino acids, and in proportions similar to those generated by the Miller
and Urey experiments. Most recently, it has been suggested that a Martian
meteorite collected in Antarctica contains evidence that life existed
on that planet. Obviously, this question will continue to be debated for
many years to come.
How did the first macromolecules and cells form?
If polymerization in an aqueous environment does not seem likely, how
did the first macromolecules form? It has been shown that polymers can
be created in the laboratory without biological catalysts. In these
experiments, dilute solutions of monomers, such as amino acids or nucleotides,
are placed on a hot, mineral substrate, such as clay or sand. The water
vaporizes and short polymers will form. It also has been shown that
macromolecules will aggregate in solution. Microspheres, which are small
droplets composed of protenoids (short polymers of amino acids), have
been synthesized in the lab. These do show some properties of cells,
including selective permeability.
How did early life transmit genetic information?
For many years, scientists were puzzled by a seeming paradox. DNA carries
genetic information and the information is used to direct the synthesis
of proteins within a cell. However, in order for this message to be
transcribed from the DNA, or for the DNA to replicate, proteins are
required. This leads to the question of which came first -- DNA or proteins?
In the 1980s, it was discovered that RNA, in addition to carrying genetic
information, could, as "ribozymes", catalyze some basic reactions. This
raised the possibility that perhaps the early earth was an RNA
World, with RNA both carrying genetic information and catalyzing
chemical reactions. This also is supported by the many functions of
RNA within the cell; it is integral to all aspects of protein synthesis,
including the formation of the peptide bond.
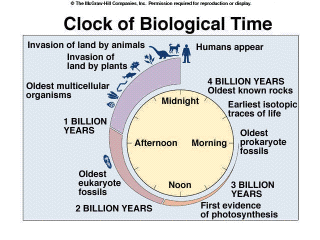
When did life originate?
The first prokaryotic fossils date to approximately 3.5 billion years
ago. Many of these earliest fossils are microfossils, requiring a microscope
to detect them. There are fossils of structures that look very much like
present day stromatolites.
These stromatolites are formed by mats of cyanobacteria, and are only
found in very saline marine environments from which grazing animals are
excluded. There are some chemical fossils dated to 3.8 billion years that
suggest biological activity because they are enriched in the isotope of
carbon, C12, that is used by living organisms. However, because there
are relatively few rocks older than 3.5 billion years, fossils of the
very earliest organisms may never be found. But, from the available evidence,
it is likely that life originated and diversified within a 500 million
year period; that earliest life was probably prokaryotic, and anaerobic.
Aerobic respiration and the eukaryotic cell arose about 1 billion years
later. In the next lesson, we turn our attention to cell structure, focusing
on the eukaryotic cell.
|