Atoms
The reason that we cover chemistry in a biology course is because all life obeys chemical principles. We will start with the basic unit, the atom. An element is the simplest form of matter and each element has a unique set of properties. An atom is the smallest component of an element that still has all of the properties of the element. An atom is to an element as a cell is to life. There are subatomic particles within an atom but if you look only at those particles you don't see all of the properties of the element that the atom belongs to. Similarly, a cell can be broken down into its components but those subcellular components don't have the properties of life. The following figure represents a helium atom. Helium exists as a gas; we use it to fill balloons, among other things.
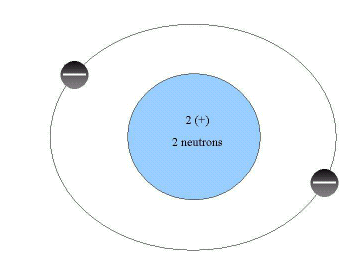
Figure 2.1. A Helium Atom
In the center of an atom, is the nucleus, which is the core of the atom. The nucleus contains most of the mass of the atom. It is composed of positively charged subatomic particles, protons(+), and neutrally charged particles, neutrons. The nucleus is orbited by electrons which have a negative charge. Electrons contribute very little weight to an atom. In the text, the author uses the analogy that if you take all the electrons in your body compared to your body weight they would weigh less than your eye lashes. While electrons have very little mass, they are very important in chemical reactions. The configuration of the electrons determines how atoms interact with each other. Helium has two protons and two neutrons in the nucleus. It has two electrons orbiting the nucleus. Here the charge of the protons is balanced by the charge of the electrons. There are two positive charges for the protons and two negative charges for the electrons. Therefore, helium doesn't have any net charge- it is a neutral atom. Atoms can be seen using a scanning tunneling electronic microscope.
The atomic number, which is the number of protons that an atom has, identifies that atom as being a specific element. For example, carbon atoms always have six protons so its atomic number is 6. The weight of an atom is the number of protons plus the number of neutrons because both subatomic particles have mass. Thus, carbon’s atomic weight is 12. Helium has an atomic number of two and an atomic weight of four because it has two protons and two neutrons. Chemists arrange the elements in a table by their atomic number and by their atomic weight.
The periodic table allows you to make predictions about how atoms should behave. This is important information if you're looking at the chemical properties of atoms. For example, if you look at the far right on the table, the column of elements, Helium (He), Neon (Ne), Argon (Ar), etc. are the noble gases. These are elements that exist as gases under normal conditions and they are very stable elements. While about 112 elements have been named, only 92 of these are naturally occurring elements; the others have been synthesized in the laboratory. Not all of these naturally-occurring elements are found in living organisms. Living organisms use a fairly small subset of all of the known elements, in particular carbon, hydrogen, oxygen, nitrogen, phosphorus and sulfur.
To continue to the next page, click on next at the top or bottom of the page (on the right side.)
