Water
The one molecule that is considered to be essential to life is water. Water has a number of special properties that are key to its central importance for biological organisms. Most of these properties stem from the distribution of the electrons that oxygen and hydrogen share in the covalent bonds that form a water molecule. Oxygen is an electronegative atom. As we discussed above, it attracts electrons to it because oxygen has a larger positive charge (8 protons in the nucleus) when compared with the hydrogen atoms (one proton in the nucleus). Therefore, the electrons spend more time orbiting around the oxygen atom than the hydrogen atoms. Because of this, different parts of a water molecule have different charges, with the oxygen having a slightly more negative charge and the hydrogen a slightly more positive charge (Figure 2.5).
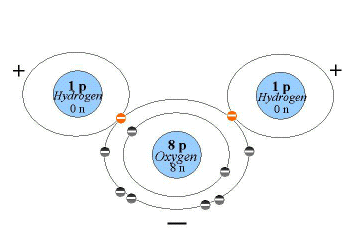
Figure 2.5. A Water Molecule
This difference in polarity means that water is a polar molecule. Hydrogen bonds join water molecules together, with the slightly positive hydrogen of one water molecule bonded to the slightly negative oxygen of anothr through weak electrostatic forces. It is this hydrogen bonding between water molecules that gives water many of its special properties.
Because of these bonds, water molecules tend to stick together, a property called cohesion. Surface tension is a consequence of this. Instead of spreading out flat on a surface, a drop of water will "bead-up". Some insects can use this property to allow them to walk on water. Figure 3.12 in you textbook shows a water strider pausing on top of the water.
Water also shows adhesion, which means that water molecules will also bond with other polar molecules. Capillary action, in which water can rise against gravity, is a result of adhesion. Plants are able to transport water from their roots to their leaves, sometimes over hundreds of feet, because water forms a column in the xylem, a type of plant vascular tissue, through adhesion with the cells of the xylem.
The difference in charge means that water can act as a polar solvent, so other molecules that are charged will dissolve in water. This is why salt dissolves when you pour it into a glass of water. The slightly positively charged hydrogen of the water molecules surround the negatively charged chloride ions (Cl-), while the slightly negatively charged oxygen of the water molecules surrounds the positively charged sodium ions (Na+), pulling them apart (Figure 2.6). Thus, the salt "dissolves". Molecules that will dissolve in water are called hydrophilic (water-loving) .
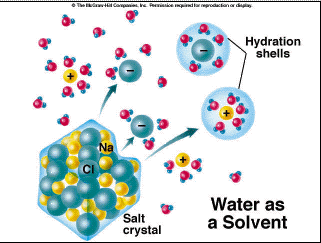
Figure 2.6. How NaCl dissolves in water.
©The McGraw-Hill Companies, Inc. Permission required for reproduction or display.Usually, as molecules go from a gas to a liquid and then to a solid, their density increases. However, solid water, or ice, is less dense than liquid water. This is a result of the hydrogen bonds between water molecules. As water freezes, the hydrogen bonds "set" the water molecules a certain distance apart, preventing them from moving closer together. Because the water molecules in ice are farther apart than they are in liquid water, ice is less dense and will float. This makes it possible for organisms that live in aquatic environments, primarily freshwater, to survive during the winter when the water freezes. They can live under the surface of the ice, where the temperature is around 4 degrees centigrade. If ice were more dense, the water would freeze from the bottom up.
Water also has a high heat capacity. A large amount of energy is required to disrupt the hydrogen bonds between water molecules and raise the temperature of water. This is important for organisms, which average about 70% water in their body composition, because it means that they are able to maintain a fairly constant temperature. Because of these hydrogen bonds, water also has a high temperature of vaporization. It takes a lot of energy to convert water from a liquid state to a gas. Many organisms take advantage of this by using evaporative cooling. When a dog pants, the evaporation of the water from its tongue disperses body heat, thus cooling the dog.
There are other properties of water that do not result directly from its polarity. Water is transparent. This means that light can penetrate it. This is important in aquatic ecosystems, because photosynthetic organisms require light energy. The region through which light can penetrate in a body of water is called the photic zone.
Water also is able to ionize, which means that the covalent bonds joining the oxygen and hydrogen break. This results in the formation of a hydrogen ion (H+) and a hydroxide ion (OH-). This is not common, as the covalent bonds joining the atoms are strong, but it does occur spontaneously. Approximately only one water molecule out of 550 million is ionized at any one moment. This corresponds to a concentration of H+ of 10-7 mole per liter in pure water. Therefore, pure water has a pH of 7.
Because the concentration of H+ and OH- are equal in pure water, pH7 is considered to be neutral. If the concentration of H+ in solution is higher, the solution is said to be acidic. Coffee (black) has a pH of 5, which means that the concentration of H+ is higher than the concentration of OH- ions. Because the pH scale is logarithmic, the coffee has a concentration of H+ 100 times higher than that of pure water. If there are more OH- in solution, then the solution is said to be basic. Bleach has a pH of about 12, which means that it has a concentration of OH- 100,000 times higher than pure water. As solutions become more acidic or more basic, they are more chemically reactive. Figure 3.14 in your textbook shows the pH scale.
To continue to the next page, click on next at the top or bottom of the page (on the right side.)
