Lipids
Lipids have a number of different functions within cells. They are energy storage molecules, they are a major component of the plasma membrane, and they also function as hormones. So, how can lipids carry out these different functions? They are not polymers in the sense that we have discussed for proteins and carbohydrates. The fundamental structure of a lipid is comprised of fatty acids and a glycerol molecule. Fatty acids are a type of hydrocarbon, long chains of carbon atoms bonded to hydrogens. Because electrons usually are distributed evenly around these molecules, they are nonpolar, or hydrophobic (water-fearing), and therefore not water soluble. We will discuss the major groups of lipids and how this structure is modified to perform different functions. |
||
Fats are used to store energy within their chemical bonds. They also serve as insulation in organisms that maintain a constant body temperature, such as mammals and birds. The building blocks of fats are triacylglycerol molecules. These molecules are composed of one glycerol and three fatty acids. The fatty acids are bonded to the glycerol molecule through three dehydration synthesis reactions, forming a triacylglycerol (Figure 2.15). |
|
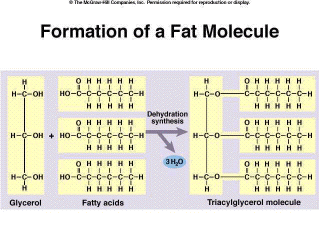
Figure 2.15. Formation of a Fat Molecule |
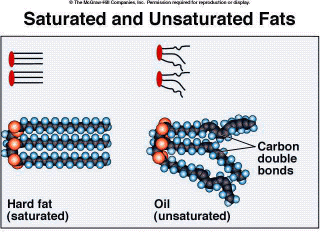
Fatty acids determine the type of fat molecule, and can differ in the number of carbon atoms and the number of carbon to carbon double bonds. Steric acid is an example of a saturated fatty acid. It has no double bonds within its hydrocarbon chain; each carbon has the maximum number of hydrogens covalently bonded, it is “saturated”. Because of this, the chains lie flat (Figure 2.16). Saturated fats are usually solid at room temperature and usually come from animal sources. Linoleic acid is an example of an unsaturated fatty acid. There are double bonds between some of the carbons within the hydrocarbon chain. These [some] carbons are not bound to the maximum number of hydrogens possible, or are unsaturated. Because of this, the chains tend to bend and flex (Figure 2.16). Therefore, unsaturated fats are usually liquid at room temperature. and These usually come from plant sources, such as corn, olives, and soybeans. Studies have shown that a diet high in saturated fats can raise the level of LDL cholesterol in the blood. Read "The Facts on Fats" on the Discovery Health Web site for more information on fats in the diet. Fats are used for energy storage in both plants and animals. The average human has about 36 pounds of fat distributed throughout their body. Fat is also used for insulation. This is especially important in marine animals, because body heat is easily lost to the water (hypothermia). Therefore, mammals such as whales and dolphins have a thick layer of blubber, which insulates their bodies and greatly reduces heat loss. |
|
Figure 2.16. Saturated and Unsaturated Fats |
Phospholipids are the molecules that comprise the basic structure of all plasma membranes. We will go into much more detail about these membranes in lesson 3. In this lesson, we will concentrate on how the structure of these molecules leads to their function within the membrane. Phospholipids have the same basic structures as triacylglycerols. However a phosphate group is present instead of the third fatty acid (Figure 2.17). This phosphate group has a negative charge. Because of this, the two ends of the molecule have different properties. The fatty acid end, with its long hydrocarbon tail, is nonpolar, and therefore insoluble in water. However, the end with the phosphate group is polar, therefore it is water soluble. When these molecules interact to form a membrane, they form two layers, with the nonpolar fatty acid tails of each layer on the inside of the membrane and the polar phosphate groups on the outside. We will leave the discussion of the complexity of the membrane until lesson 3. |
|
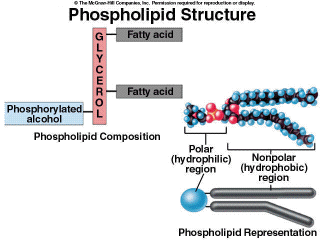
Figure 2.17. Phospholipid Structure |
Steroids are the group of lipids that includes many hormones. These molecules are secreted from glands that are part of the endocrine system and are used for long-range communication within the body. We will discuss the action of hormones when we cover human reproduction in lesson 10. Testosterone and estrogen are steroid hormones. These molecules generally have more complex ring structures, as shown in Figure 2.18. Another type of steroid is cholesterol. Although excess levels of LDL cholesterol have been associated with coronary disease in human, this molecule is an integral part of the plasma membrane in most animal cells. Pigments are another important group of steroids. We will discuss the pigment chlorophyll in lesson 4. |
|
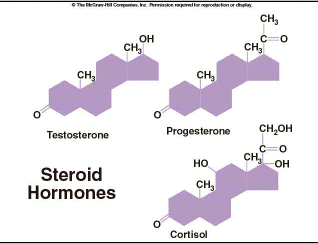
Figure 2.18. Steroid Hormones |
To continue to the next page, click on next at the top or bottom of the page (on the right side.)