Chemical Bonding
The process of the interaction between atoms is called bonding. There are several types of bonds depending upon the number of electrons in the atom. These different bonds are important in the structure and functioning of the various molecules. Because bonding depends upon the chemical properties of these atoms, it is important to look at the configuration of the electrons. Your text gives a good description of the position of electrons in an atom. If you think about the nucleus which consists of the protons and neutrons, being about the size of apple, the electrons would be about a mile away. They are like a "cloud" swarming around this atom. However, to represent them we draw them as orbitals. The first orbital can hold a maximum of two electrons. Each successive orbital can have eight electrons. Figure 2.2 shows two elements, Helium and Neon. Helium has two electrons in its first orbital, while Neon has two electrons in its first orbital and eight electrons in the second orbital. These orbitals are full, carrying the maximum number of electrons for each level, which is why these gases are not chemically reactive. They do not form bonds with other atoms. Atoms that do not have a full outer orbital, however, will forms bonds in order to achieve that stable state.
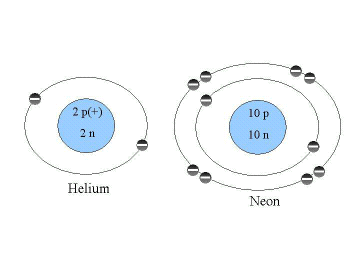
Figure 2.2. The Configuration of Electrons in Helium and Neon
We make this pictorial representation of electrons, but remember that electrons are never in the same position; they are constantly moving around the atom. Electrons have different energy states. If the electron is zipping around the atom and is fairly close to the nucleus, it's at a low energy level. The farther away the electron is from the nucleus, the more energy it is holding, so the higher the orbital, the more energy the electron has (see Figure 3.2 in your text). These orbitals and the energy in electrons, will be important when we talk about some of the cellular processes, such as photosynthesis. As I mentioned earlier, what we are most interested in is how individual atoms interact with each other and how atoms form molecules.
Molecules and Compounds
In Biology we usually don't talk about single elements, we talk about molecules and compounds. A compound is made up of atoms of two or more elements in a fixed ratio held together by chemical bonds. A molecule is the smallest unit of a compound. A molecule is to a compound as an atom is to an element. For instance, water is a compound composed of molecules of H2O, which means it has two hydrogen atoms and an oxygen atom. These atoms are bonded together in a specific way that gives water some unique properties. You can write the formula as H2O, or your can write it H-O-H, showing the position of the atoms relative to each other. As mentioned earlier, the physical characteristics of a compound are not necessarily the same as the physical properties of the elements which it is composed of. Using water as an example, we have hydrogen, which is a gas, and oxygen, which is a gas, making a liquid. Once these atoms are bonded together they exhibit different physical properties. While under what we call standard conditions, water is a liquid, we also all know that water can become a solid and water can become as well as a gas. Salt, NaCl, is another compound, composed of sodium (Na) and chloride (Cl) atoms. The molecules that comprise compounds are formed by chemical bonds between atoms. There are three types of chemical bonds that we will focus on, ionic bonds, covalent bonds and hydrogen bonds.
Ionic Bonds
Atoms are most stable when the have a complete outer electron shell. Because of this, atoms which have only one or two electrons in their outer orbital easily lose them to a atom which only needs one or two electrons to complete its outer orbital. The result is that each now has a full outer shell but they are left with a net electrical charge. Atoms which have an unequal number of protons and electrons are called ions. Ionic bonds then form between the ions due to the attraction of the opposite charges. For example, sodium chloride (NaCl) is formed through an ionic bond between a sodium ion and a chloride ion. In this process, Na loses the single electron in its outer orbital to Cl which had 7 electrons in its outer orbital. Na is now positively charged and Cl negatively charged. These opposite charges attract, holding the atoms together through electrostatic force (Figure 2.3).
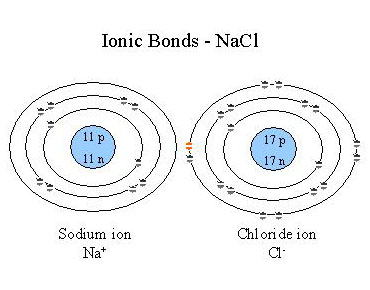
Figure 2.3. Ionic Bonds - NaCl
Covalent Bonds
Covalent bonds form when electrons are shared between atoms. Essentially, this means that the electrons are orbiting around both of the atoms. This combining of orbitals results in a very strong and stable bond. The biological macromolecules that we are going to study are largely held together by covalent bonds. Water is an example of a molecule that is formed through covalent bonds. The oxygen atom has six electrons in its outer shell, so there is room for two more electrons before that shell is full. Each hydrogen atom has one electron in its electron shell and needs one more to be full (remember that the first orbital only holds two electrons). The two hydrogen atoms each share their electron with the oxygen atom (Figure 2.4), essentially filling the outer orbital of all three atoms. Carbon dioxide (CO2) is another example of a compound in which the atoms form molecules through covalent bonds. In this case, carbon needs four electrons and a double bond is formed between the carbon and each of the oxygen. In a double bond two electrons are shared between the two atoms.
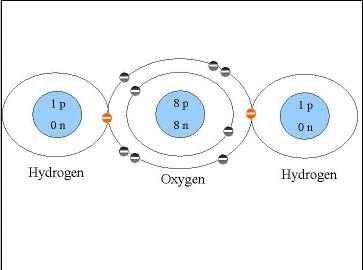
Figure 2.4. Covalent Bonds - H2O
Hydrogen Bonds
Another type of bond that is extremely important in biological systems is the hydrogen bond. These are very weak bonds that form between polar molecules. Polar molecules occur when one of the atoms in a covalent bond has a stronger attraction for the shared electron than the other. This usually involves an oxygen or nitrogen bonded with hydrogen. The result is that the electron spends more time around the larger atom giving that end of the molecule a partial negative charge and leaving the hydrogen atom with a partial positive charge. Hydrogen bonds then form between the slight negative end of one molecule and the slight positive end of another molecule. Hydrogen bonding is an important property of water as we’ll discuss next and in the structure and function of proteins and nucleic acids (DNA and RNA).
To continue to the next page, click on next at the top or bottom of the page (on the right side.)
