Proteins
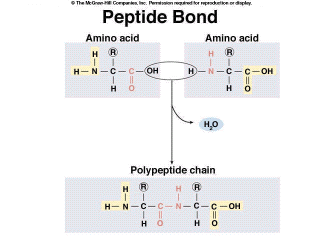
Proteins have many diverse functions within organisms. See Table 3.4 in your textbook. Proteins are polymers of amino acids. Organisms use about 20 different amino acids to make proteins, and this means that the possible sequences of amino acids within proteins are almost infinite. There are structural proteins, such as the keratin that gives support to your skin or to a bird's feather. Enzymes are proteins that catalyze chemical reactions within a cell, such as amylase which breaks starch into disaccharides and DNA polymerase which joins together DNA monomers. Some proteins transport substances across cell membranes, or through the body. For example, hemoglobin transports oxygen through your circulatory system. There are even some proteins that function as hormones, including insulin, which regulates the level of glucose in your blood. All proteins have the same basic structure. Amino acids are the monomers of proteins (Figure 2.12). They consist of a central carbon atom, which is bonded to a carboxyl group (COOH) at one end and an amino group (NHH) at the other. The carboxyl group allows the molecule to act as an acid, donating H+, while the amino group allows it to act like a base, accepting H+ ions. The central carbon is covalently bonded to a side chain, the functional or “R group”. This R group is different for each of the 20 common amino acids and determines the property of the amino acids. R groups range from a single hydrogen atom to complex ring structures. Amino acids are joined together by dehydration synthesis to form a molecule called a polypeptide. When two amino acids are covalently bonded together, the bond is called a peptide bond, hence the name polypeptide (Figure 2.12). |
|
Figure 2.12. Amino Acid Structure and Formation of the Peptide Bond |
Most proteins do not function as a simple linear polypeptide chain.[, which is their primary structure] In order to perform a specific task, they must be folded into a specific shape (Figure 2.13). There are four levels of protein structure. The first level, their primary structure, is the sequence of the amino acids. Secondary structure is local folding [, usually through] which is held in place by hydrogen bonding of amino acids in close proximity to each other (Figure 2.13).
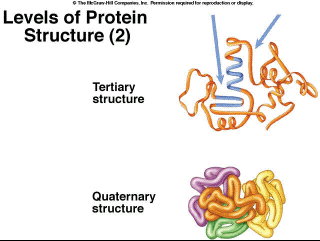
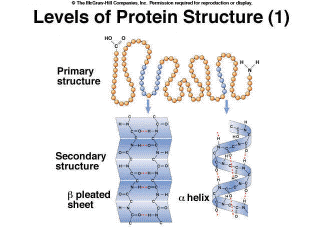 Figure 2.13. Levels of Protein Structure (1) Tertiary structure involves folding of the molecules across longer distances (Figure 2.14), often through disulfide bridges, which are bonds between two amino acids that contain a sulfur atom. These bridges occur in the proteins in hair. If you go the the hairdresser and get a permanent, the tertiary structure is denatured chemically. Sulfur atoms are released, resulting in the characteristic odor. The hair is then treated with other chemicals that reform the bonds when the hair is on rollers, giving it a different shape, curly versus straight. Finally, quaternary structure is the association of multiple polypeptides (Figure 2.14). For example, the protein hemoglobin consists of four polypeptides joined together.
Figure 2.14. Levels of Protein Structure (2) There are special proteins called chaperone proteins which aid in the proper folding of the polypeptides into their final shape. Some assist in the folding of newly made proteins while others appear to refold proteins which are misfolded or have unfolded (denatured) due to increased temperatures. |
||
To continue to the next page, click on next at the top or bottom of the page (on the right side.)