Forming Macromolecules
All organisms are composed of biological macromolecules. These are carbon-based compounds, which means that they have a fundamental structure composed of carbon atoms. Why carbon? Because carbon has only four electrons in its outer shell, it can form covalent bonds with four other atoms. Thus, it is able to form very stable bonds with other carbon molecules, enabling the formation of long carbon chains. Figure 2.7 shows methane,CH4, a simple carbon compound formed by these covalent bonds.
|
|
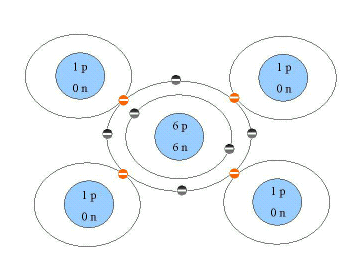
Figure 2.7. Methane |
Biological macromolecules generally are polymers, (poly = many; mer = unit), formed by joining monomers, or single molecules, together in a long chain. They are formed by the process of polymerization. In this process, dehydration synthesis, or the removal of a water molecule, joins two monomers together (Figure 2.8). When cells produce polypeptides, chains of amino acids, during protein synthesis, the reaction that joins these amino acids together is a dehydration reaction (also called a condensation reaction). Water molecules can be inserted between monomers to break down a polymer in a reaction called hydrolysis (Figure 2.8). This happens during digestion. For example, when you eat a cracker, enzymes in your mouth catalyze a chemical reaction, hydrolyzing the starch (a polymer) in the cracker and breaking it down into more simple sugars. This is why a cracker begins to taste sweet after you chew it. |
|
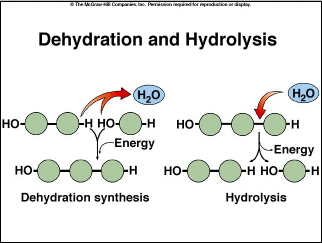
Figure 2.8. Dehydration and Hydrolysis |
There are four major classes of biological macromolecules (Figure 2.9). The carbohydrates have two primary functions, to provide energy for cells and to provide structure. Proteins play many roles in a cell, from enzymes which catalyze chemical reactions such as the amylase secreted by your salivary glands, to providing structure, the role of the keratin in your skin. Some proteins, such as insulin, function as hormones. Lipids also have a diversity of functions, including energy storage as well as forming the primary structure of cell membranes. Nucleic acids are information storage molecules, and play a role in converting this information into polypeptides. These biological macromolecules can be very large. For example, the chemical formula of bovine growth hormone is C999H1529N263O299S7! |
|
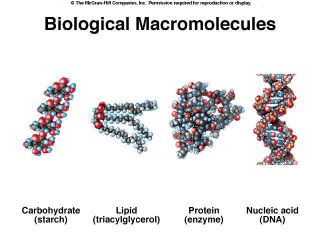
Figure 2.9. Biological Macromolecules |
To continue to the next page, click on next at the top or bottom of the page (on the right side.)
